 “I am very pleased to report that demand for the Dendrite Web-Registry platform has reached an all-time high” Dr Peter Walton, Managing Director of Dendrite Clinical System. “We’re receiving new orders for new national and international registries every few days now – for surgical registries, medical registries, rare disease registries, medical device registries and Coronavirus Registries (for a range of Community, National, International and Global Covid-19 Registries).”
“I am very pleased to report that demand for the Dendrite Web-Registry platform has reached an all-time high” Dr Peter Walton, Managing Director of Dendrite Clinical System. “We’re receiving new orders for new national and international registries every few days now – for surgical registries, medical registries, rare disease registries, medical device registries and Coronavirus Registries (for a range of Community, National, International and Global Covid-19 Registries).”
“Over 90% of our new web-registry orders include our innovative e-PROMS module that enables patients to enter data into registries remotely”. The automated e-PROMS module can automatically send (at designated time intervals) a secure personalised message to appropriately consented patients and they can complete a PROMS questionnaire on their smart-phones, iPads or computers anywhere in the world (provided they have Internet access) and when completed, the data returns automatically to the registry.
“We have instituted over 35 different PROMS instruments ranging from generic outcome Euroqol EQ-5D type questionnaires, to a very wide range of disease or treatment specific PROMS instruments such as the Breast Q for breast reconstruction with DIEP/SIEA flap, the Atrial Fibrillation Severity Score and the Multiple Sclerosis Quality of Life MSQoL-54 questionnaire,” Dr Walton added. “A great benefit of our e-PROMS module is that essentially the patient is doing the work of data collection and the data just streams in. The e-PROMS module is guaranteed to provide safe and secure patient data collection, and we look forward to working with all our current and future customers to help them develop and expand PROMS data collection.”
Patient Reported Outcome Measures (PROMs) are standardised, validated instruments or questionnaires that are completed by patients to measure their perception of their functional well-being and health status, and patients rate their health by scoring the severity or difficulty in completing certain tasks or routine activities. Dendrite’s flexible innovative e-PROMS module can be adapted include bespoke instruments across the whole spectrum of clinical scenarios, whilst maintaining patient anonymity and confidentiality, ensuring data validation, increasing efficiency and simplifying the data collection process).
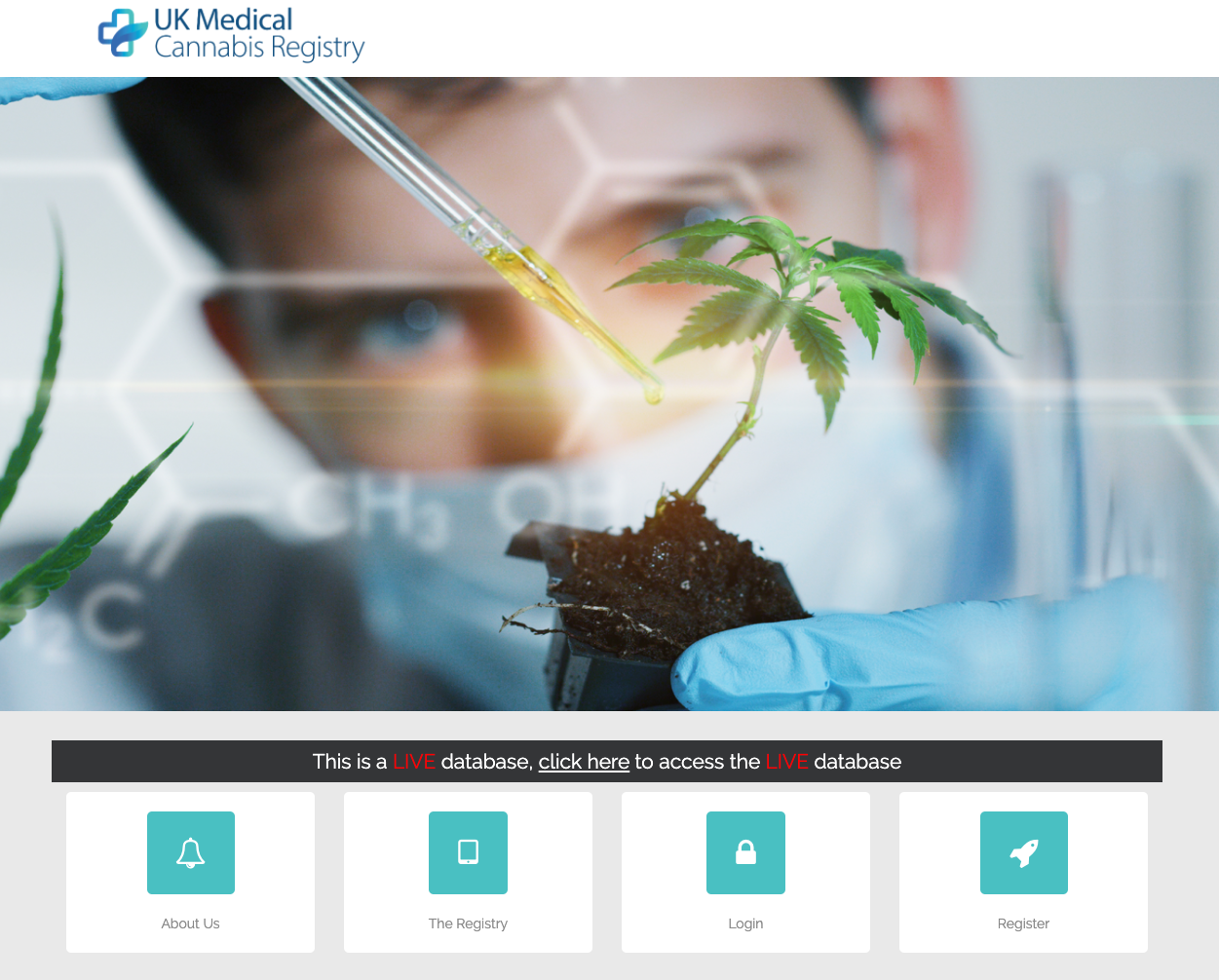 Sapphire Medical Clinics and Dendrite Clinical Systems have launched the UK Medical Cannabis Registry, an initiative that is designed to rapidly expand the evidence base for medical cannabis in the UK and decrease the cost of access for patients. The launch will collect and analyse clinical information on patients taking medical cannabis treatments for all recognised eligible conditions.
Sapphire Medical Clinics and Dendrite Clinical Systems have launched the UK Medical Cannabis Registry, an initiative that is designed to rapidly expand the evidence base for medical cannabis in the UK and decrease the cost of access for patients. The launch will collect and analyse clinical information on patients taking medical cannabis treatments for all recognised eligible conditions. Sapphire Medical Clinics and Dendrite Clinical Systems have established the UK’s first national UK’s first comprehensive medical cannabis patient registry - UK Medical Cannabis Registry. The UK Medical Cannabis Registry will be the first of its kind covering all conditions for which there is evidence of clinical efficacy of medical cannabis. The formation of the registry means that real world evidence, cited as a key barrier to wider patient prescriptions by NHS England, will now be available on request to the medical community for analysis.
Sapphire Medical Clinics and Dendrite Clinical Systems have established the UK’s first national UK’s first comprehensive medical cannabis patient registry - UK Medical Cannabis Registry. The UK Medical Cannabis Registry will be the first of its kind covering all conditions for which there is evidence of clinical efficacy of medical cannabis. The formation of the registry means that real world evidence, cited as a key barrier to wider patient prescriptions by NHS England, will now be available on request to the medical community for analysis. The British Association of Plastic, Reconstructive and Aesthetic Surgeons (BAPRAS), in conjunction with Dendrite Clinical Systems, is delighted to announce the release of the First UK National Flap Registry (UKNFR) Report. The UKNFR, which was launched in August 2015, collects information on all major free and pedicled flap operations carried out in the UK.
The British Association of Plastic, Reconstructive and Aesthetic Surgeons (BAPRAS), in conjunction with Dendrite Clinical Systems, is delighted to announce the release of the First UK National Flap Registry (UKNFR) Report. The UKNFR, which was launched in August 2015, collects information on all major free and pedicled flap operations carried out in the UK. Dendrite Clinical Systems, the publisher of Bariatric News, has announced the publication of the Bariatric News Industry and Product Guide 2019 – a comprehensive A-Z guide to companies, products and services within the bariatric and metabolic specialty.
Dendrite Clinical Systems, the publisher of Bariatric News, has announced the publication of the Bariatric News Industry and Product Guide 2019 – a comprehensive A-Z guide to companies, products and services within the bariatric and metabolic specialty.  Dendrite Clinical Systems, the publisher of Bariatric News, is pleased to announce issue 39 of the newspaper is now available to view/download. The newspaper reports on research, technology, events and policy in the bariatric specialty, the latest clinical studies, policy changes and product news, the latest meetings and events, interviews prominent bariatric experts, and host debates between specialists on controversial topics.
Dendrite Clinical Systems, the publisher of Bariatric News, is pleased to announce issue 39 of the newspaper is now available to view/download. The newspaper reports on research, technology, events and policy in the bariatric specialty, the latest clinical studies, policy changes and product news, the latest meetings and events, interviews prominent bariatric experts, and host debates between specialists on controversial topics.


